- Silicones are a class of synthetic inorganic polymers consisting of a polymer chain of silicon-oxygen-silicon atoms linked together
- Because of their general thermal stability, good electrical insulation characteristics, constancy of properties over a wide temperature range, water-repellency and anti-adhesive properties, the silicone polymers find use in a very wide diversity of applications
- Polymers are available in a number of forms such as fluids, greases, rubbers and resins.
In 1904 Professor F.S. Kipping, discovered the principle of intermolecular condensation of silane diols polymerisation.
Term silicone was given by Kipping to the hydrolysis products of disubstituted silicon chlorides
In 1946 the General Electric Company, NY, started production of silicone polymers
Union Carbide Corporation started production of silicones in 1956.
During the 1970s growth rates for silicones were higher than for many other commercial polymers.
PREPARATION OF METHLYL/PHENYLCHLOROSLINE:
The Grignard Method
- Because of the differences in the reactivities of the intermediates a high yield of dichlorodimethylsilane is produced.
- Products are recovered by fractional distillation.
- Grignard method was the first route used commercially in the production of silicone intermediates
The Direct Process
- Process is operated in a fluid-bed system on a continuous basis.
- Silicon metal is crashed and ground to a fluidizable particle size range and mixed with finely divided copper catalyst
Flow diagram for methyl chlorosilane production.
MANUFACTURE OF SILICONE POLYMERS
- A variety of silicone polymers has been prepared ranging from low-viscosity fluids to rigid cross-linked resins.
Flow sheet of a silicone manufacturing facility
(Fluids, Resins, RTVs and Heat Cured Rubbers)
SILICONE FLUIDS: Silicone fluids form a range of colourless liquids with viscosities from 1 to 1,000,000 centistokes
Properties: Dimethylsilicone fluids are colourless, odourless, of low volatility and non-toxic.
- They have a high order of thermal stability and a fair constancy of physical properties over a wide range of temperature (-70°C to 200°C)
SILICONE RESINS
Preparation
- Silicone resins are prepared batch-wise by hydrolysis of a blend of chlorosilanes.
- Trichlorosilanes must be incorporated into the blend to get cross linked silicone resin.
- Chlorosilanes are dissolved in suitable solvent and blended with the water which may contain additives to control the reaction.
- At the end of the reaction the polymer-solvent layer is separated from the aqueous acid layer and neutralized.
- Heating the resin with a catalyst such as zinc octoate at 100°C to get desired viscosity value.
Properties : Silicone resins have very good heat resistance
SILICONE RUBBER
- In spite of high cost, silicone rubbers are used in variety of applications where heat resistance and retention of properties over a wide range of temperatures are required
Dimethylsilicone Rubbers: Elastomers consist of very high molecular weight (~0.5 X 106) linear gums cross-linked after fabrication.
- Difunctional monomers be employed to achieve high molecular weight resin.
- Modified polydimethylsiloxane Rubbers
- Dimethylsilicone rubbers show a high compression set which can be reduced by additives mercurous oxide and cadmium oxide.
- Substantially reduced compression set values may be obtained by using a polymer containing small amounts of methylvinylsiloxane (~0.1 %)
ROOM TEMPERATURE VULCANIZING SILICONE RUBBERS
(RTV RUBBERS)
- RTV silicone rubbers may be classified into two types
Two-Pack Systems- RTV – 2 Rubbers:
- Two component system can be designed by the polymers and curing agent are in one package and the catalyst alone or the curing agent and the catalyst in the other.
- Cure is triggered by mixing the contents of the two packages.
- Fillers and additives are incorporated in the formulations according to the desired product properties.
- The two-pack systems may be subdivided into
- Condensation cross-linked materials.
Addition cross-linked polymers.
- Condensation system involves reaction of a silanol-terminated polydimethylsiloxane with a multi-functional organosilicon cross-linking agent such as Si(RO)4
ONE-PACK SYSTEMS (RTV- I RUBBERS)
- RTV-l rubbers are produced by first producing a polydialkylsiloxane with terminal hydroxyl groups, then reacted with a multi-functional organosilicon cross-linking agent of the type RSiX3, where X may be
-NH-R (amine) , -O-(CO)-CH3 (acetate), -O-N=C(R2) (oxime)
- Si-X linkages react with water to form a Si-O-Si linkage with the liberation of HX
- Catalysts include diaryl alkyl tin acylates
- Curing reaction may be brought about by atmospheric humidity and such rubbers are also known as moisture-curing silicones
Flow diagram for continuous one-component RTV processing.
COMPOUNDING
- Before fabrication it is necessary to compound the silicone rubber (gum) with fillers, vulcanizing agent and other special additives on a two-roll mill or in an internal mixture.
- Incorporation of fine fillers is necessary if the vulcanisates are to have high strength
- Fine silica fillers are generally used. Carbon blacks do not give outstanding reinforcement, adversely affect electrical insulation properties and may interfere with the curing action.
- Silicone rubbers are normally cured with peroxide, benzoyl peroxide, 2, 4-dichlorobenzoyl peroxide and t-butyl per benzoate - 0.5-3%.
CROSS-LINKING OF SILICONE RUBBER
- In order to develop the rubbery properties it is necessary to cross-link (vulcanize) the compound after shaping by heating in a press for 5 – 25 mints at temp range 115 to 175oC.
- Prolonged post curing at temperature upto 250 o C may be necessary in order to achieve best mechanical and electrical properties
STRUCTURE AND GENERAL PROPERTIES:
- Siloxane Si-O link has a number of interesting properties since both Si-O and Si-CH3 bonds are thermally stable. Hence polydimethylsiloxane has good thermal stability.
Moulded silicones are characterized by the following properties:
- Good dimensional stability at high temperatures,
- Good electrical and dielectric properties over wide frequency and temperature ranges,
- Low water adsorption,
- Flame resistant without additives, self extinguishing,
- Good flow properties,
- Long curing time in comparison with other moulding compounds,
- Limited shelf life,
- Average level of mechanical properties,
- High cost.
Physical properties of general purpose silicone rubber compound:
PHYSICAL PROPERTIES
- Important properties of the rubbers are their temperature stability, retention of elasticity at low temperatures and good electrical properties.
- Temperature range of general purpose material is approximately -50 to + 250°C but both ends of the range may be extended by the use of special purpose materials
- Compared with organic rubbers the silicones have a very high air permeability, being 10-20 times as permeable as the organic rubbers.
Thermal conductivity is also high, about twice that of the natural rubber.
- It is more expensive than the conventional rubbers (e.g. natural rubber and SBR)
CHEMICAL PROPERTIES
Resistance to Chemicals
- Silicones are resistant to dilute mineral acid and alkaline solutions, sea-water, methanol, glycol and formic acid but are not resistant to aromatic hydrocarbons and concentrated acids and alkalis.
Weathering Resistance
- Weathering resistance of silicone resin mouldings is same as other thermoset compounds
Resistance to High Energy Radiation
- On exposure to high-energy radiation silicone mouldings first cross-link and then decompose
Flammability
- Because of its thermal stability, silicone exhibits a higher ignition temperature than other plastics.
Toxicological Assessment
- Silicones are basically physiologically inert.
PROCESSING
- Compounded rubbers are suitable for normal processing techniques employed in rubber technology. e.g extrusion, calendaring and compression moulding.
- Pre-warmed preforms are usually transfer moulded for encapsulating electrical components.
- Mould time is 1 to 5 min at 150 to l80°C and pressures of 300 to 700 MPa.
- Processing shrinkage is low, about 0.2 to 0.9%.
AVAILABILITY
- Silicone polymers available in various forms such as oils, resins, pastes and elastomeric moulding compounds.
- It is available in form of paste as two component curing system and single component moisture cure system.
APPLICATIONS
Silicone fluids
- Silicone fluids find a very wide variety of applications mainly because of their water-repellency, anti-stick properties, low surface tension and thermal properties
Polish additives:
- Polishes contain normally 2- 4% of silicone together with a wax which has been formulated either into an aqueous emulsion or a solution in a volatile solvent.
- The value of the silicone fluid is not due to water-repellency or anti-stick properties but due to its ability to lubricate the wax plates and enable them to slide past each other.
- The effort in polishing a car with a polish containing silicone fluid is claimed to be less than half that required with a conventional wax polish.
Release agents
- Dilute solutions or emulsions containing 0.5 – 1 % of a silicone fluid have been extensively used as a release agent for rubber moulding
Fluids have also been found to be of value in the die-casting of metals
- Greases have also found uses in the laboratory for Iubricating stop cocks and for high-vacuum work.
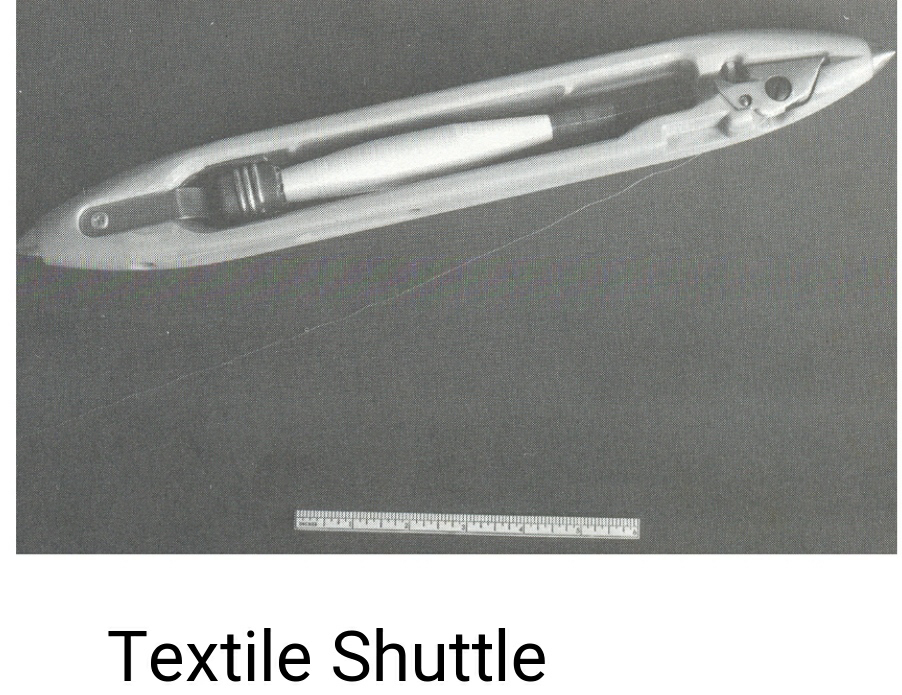
Water-repellent applications:
- Silicones have established their value as water-repellent finishes for a range of natural and synthetic textiles
- Leather may similarly be made water repellent by treatment with solutions or emulsions of silicone fluids.
- Silicone fluids containing Si-H groups are also used for paper treatment.
Lubricants and greases
- Silicone fluids and greases have proved of use as lubricants for high-temperature operation, for applications depending on rolling friction.
- Silicone greases are used primarily as electrical greases for aircraft and car ignition systems
Mould Release agent
Silicone Resins
Laminates:
- Methyl-phenylsilicone resins are used in the manufacture of heat-resistant glass-cloth laminates, particularly for electrical applications
- The glass cloth is cleaned and dipped in solution of the rsin and cured by heating
- Resin pick-up is usually in the order of 35-45% for high-pressure laminates and 25-35% for low-pressure laminates.
- A number of curing catalysts have been used, including triethanolamine, zinc octoate and dibutyl tin diacetate.
- Silicone laminates are used in electrical applications such as slot wedges in electric motors, terminal boards, printed circuit boards and transformer.
- There is also some application in aircraft, including use in firewalls and ducts.
Moulding Compositions
- Compression moulding powders based on silicone resins consist of mixtures of a heat-resistant fibrous filler (e.g. glass fibre or asbestos) with a resin and catalyst.
- They may be moulded, at temperatures of about l60°C for 5-20 minutes using pressures of 7-30 MPa.
- Used in the moulding of brush rings holders, switch parts and other electrical applications that need to withstand high temperatures.
Silicone Rubbers
- Silicone rubbers find use because of their excellent thermal and electrical properties, their physiological inertness and their low compression set.
- Specific uses include shaft sealing rings, spark plug caps, O-rings the major market for the fluorosilicones, gaskets, coolant and heater hoses for buses and trucks, and ignition cables.
- The passenger and military aircraft each use about 500 kg of silicone rubber for gaskets and sealing rings for jet engines, ducting, sealing strips, vibration dampers and insulation equipment.
- Silicone cable insulation is also used extensively in naval craft since the insulation is not destroyed in the event of a fire but forms a protective and insulating layer of silica.
- Rubbers are used as blood transfusion tubing capable of sterilization, antibiotic container closures, electric iron gaskets and domestic refrigerators.
- Silicone rubbers have been widely used for medical applications, particularly for body implants in structural cosmetic surgery.