POLYPHENYLENE OXIDE (PPO):
In 1965, A. Hay of General Electric produced polyphenylene oxide by the copper catalyzed oxidation of 2, 6 xylenol [(CH3)2 C6H3 OH)].
This is an aromatic polyester in which benzene rings is linked at the 1 and 4 position via. Oxygen atom.
The chemical formulation is based on the oxidative coupling of substituted phenols and the elimination of a molecule of water. The full chemical name of PPO is poly [1, 4 – (2, 6-dimethyl phenyl) ether].
Structurally, PPO is made of phenylene rings linked together by ether linkages in the 1,4 or para- positions, with methyl groups attached to carbon atoms in the 2 and 6 positions.
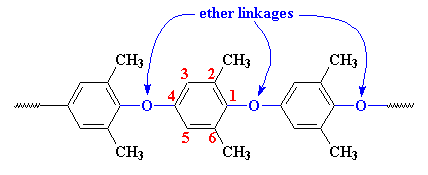
Chemistry of Preparation of PPO:
- Poly-(phenylene oxide) is known as poly (2,6-dimethyl-p-phenylene ether).
- The most successful method of preparation involves oxidative coupling of 2, 6 dimethyl phenol.
- The reaction is carried out at 28-46°C by bubbling oxygen through a solution of the phenol in the presence of a copper (I) salt-amine complex for about 7 minutes.
- The rigid structure of the polymer molecules leads to a material of a high Tg of 208°C.
- There is a also a secondary transition at 116°C and the small molecular motions that this facilitates at room temperature give the polymer in the mass a reasonable degree of toughness.
- When polymerized the polymer is crystalline but has a surprisingly low reported melting point (Tm) 257°C.
- The ratio of Tg & Tm of 0.91 (in terms of K) is uniquely high. Because of the small differences in Tg and Tm there is little time for crystallization to occur on cooling from the melt and processed polymer is usually amorphous.
- However if molecular movements are facilitated by raising the temperature or by the presence of solvent crystallization can occur.
The polymer is dissolved by halogenated and aromatic hydrocarbons of similar solubility parameter. Stress cracking can occur with some liquids.
- Being only lightly polar and well below the Tg at common ambient temperature the polymer is an excellent electrical insulator even at high frequencies.
- The polymer exhibit very good hydrolytic stability.
- One particular feature of PPO is its exceptional dimensional stability amongst the so-called engineering plastics.
- It has a low co-efficient of thermal expansion, low molding shrinkage and low water absorption thus enabling molding to close tolerances.
- It is more or less self-extinguishing polymer with a good chemical resistance properties.
Characteristics of PPO (For Identification)
- It is semi-crystalline and having melting point of 257°C
- Characterized by good dimensional stability and excellent resistance to hydrolysis and very good dielectric properties over a wide range of temperature
- Its short term and long term service temperatures are 100 and 80°C
- It is characterized by orange yellow flame with black smoke and self extinguishing character
- It melts but chars
- It smells phenolic (ink smell) while it is burnt.
Characteristics of PPO:
Possesses high melting point and glass transition temperature
- Low co-efficient of thermal expansion
- Continuos use temperature upto 100°C
- Excellent electrical properties
- Low loss factor
- Dissipation factor is quite low
- Very good dimensional stability
- High heat distortion temperature (179°C)
- Low moulding shrinkage
- Low warpage
- Good impact resistance
- Low water absorption
- Soluble only in halogenated and aromatic hydrocarbons
- Excellent flame retardancy
- Outstanding hydrolytic stability
PROPERTIES OF PPO:
1. High Heat Resistance
2. Excellent Impact Strength
3. Exceptional dielectric and dissipation characteristics
4. Flame Retardancy
5. Exceptional low moisture absorption
6. Halogen-free flame retardancy
7. Excellent dimensional stability
8. Low mould shrinkage
Blends of Polyphenylene Oxide
1. PPO/PS blends, PPO/PA blends
2. Polyphenylene Oxide is completely miscible with Polystyrene in all proportions.
APPLICATIONS:
Automotive
Fenders, dash- boards,
Head lamp systems,
Instrument and Control Panels,
Mud-guards,
Wheel Covers & Fuse Blocks etc.
Communications
Computer Housings
Television Cabinets,
Telephone and Fiber
Optic Connectors,
Video display terminals,
Control Panels,
Printer Bases etc.
Appliances
Air conditioner grills and Components
Pump housings for dish
washers & Cloth-Washers,
Handles etc..
Electrical: The electrical and dielectric properties of noryl are very good and almost independent of frequency and temperature.
- Plug and Socket Connectors,
- Electrical Wall Plates,
- Outlet boxes,
- Connectors,
- Switches etc..
Chemical Properties:
PPO. mod. is resistance to dilute acids strong alkalines, solutions, alcohols, detergents, fats and oils depending on the additives.
- It is not resistant to concentrated acids, ketones, chlorinated hydrocarbons, aromatics they swell in many aliphatics.
- PPO mod. Is susceptible to stress cracking because of the styrene content.
Weathering Resistance
- Weathering resistance of PPO mod. is less than that of the unmodified material but still relatively high.
- The long-term heat resistance in air and water in the presence of oxygen is inferior to that of polyacetals .
Flammability
Flame retardant grades are self-extinguishing and non-dripping.
Toxicity
- Correspondingly marked grades are non-toxic Grades incorporating demolding aids, UV stabilizer and glass fiber reinforcements are permitted.
- Restrictions apply only to colored grades (cadmium pigments).
- Suitable non-toxic pigments are carbon black,Ti02,ultramarine blue and iron oxide.that of polyacetals
Flammability
- Flame retardant grades are self-extinguishing and non-dripping.







No comments:
Post a Comment